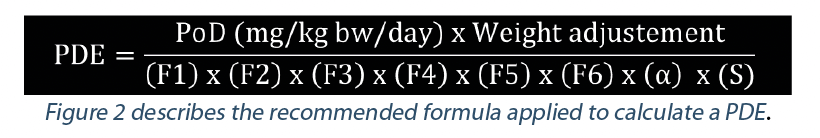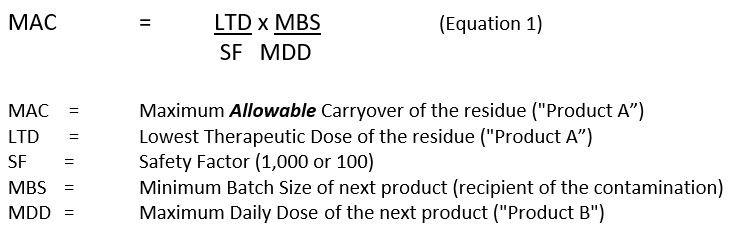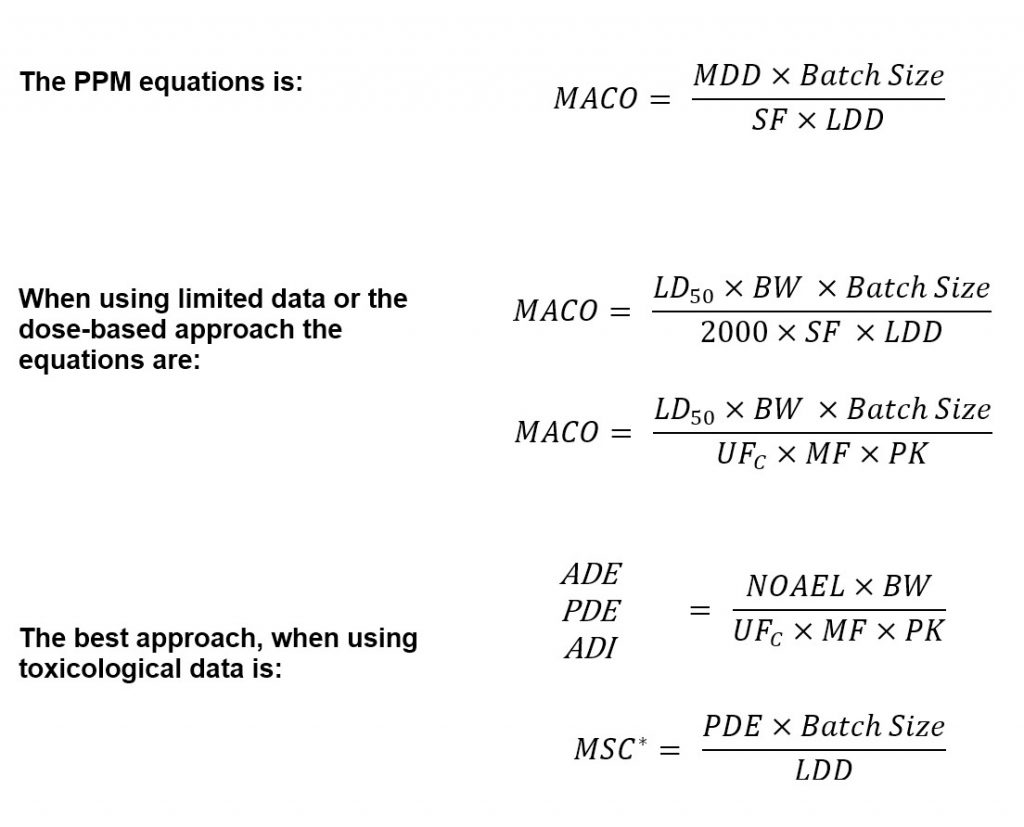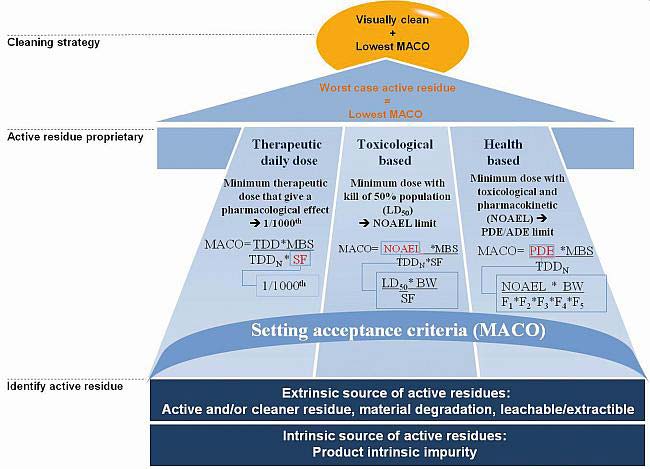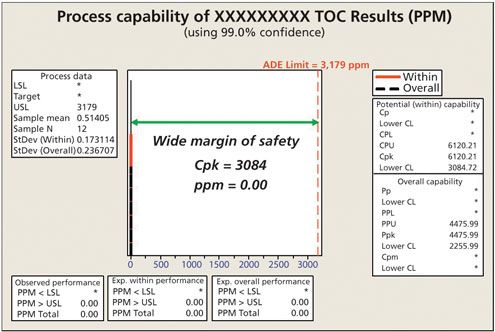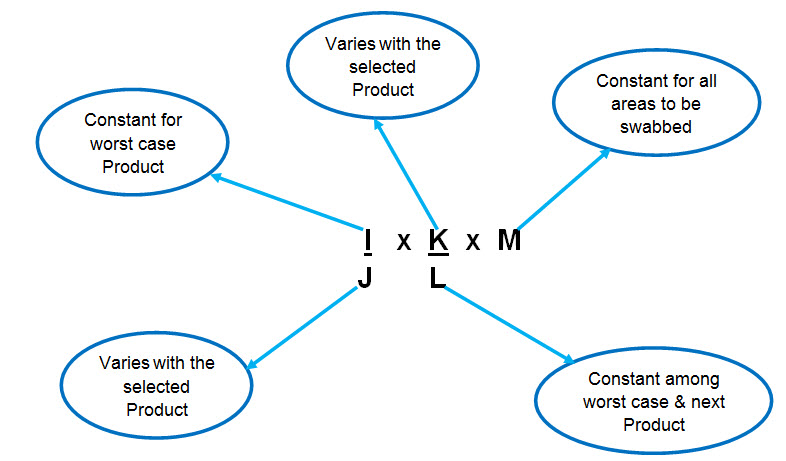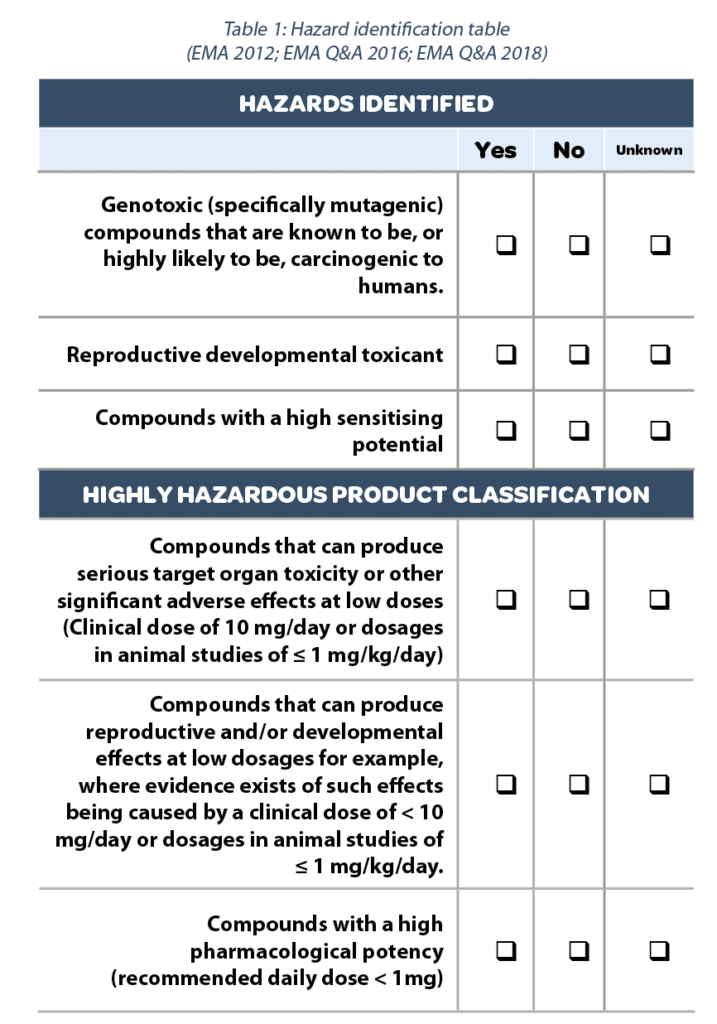
Toxicological approach to define the PDE for your cleaning validation process. - A3P - Pharmaceutical & Biotechnology Industry

PERMITED DAILY EXPOOSURE (PDE) VALUE DETERMINATION OF MEROPENEM: PDE Value for Cephalosporin Molecules for Cleaning Validation; Determination Of PDE Value of Meropenem ; NOAEL value of Meropenem: MAMUN, AL, MAMUN: 9798680186053: Amazon.com:
Case Study – Evaluation of Health-Based Exposure Limits and Potential Impact on Manufacturing Equipment Cleaning Limits Presen

PDE-Based Cleaning Limits: Best Practice or Deficiency? - GMP- und GXP Experten | Expertsinstitut Beratungs GmbH

What RDC 301/2019 requires facing the toxicological evaluation and ADE/PDE in cleaning validation · Kivalita Consulting
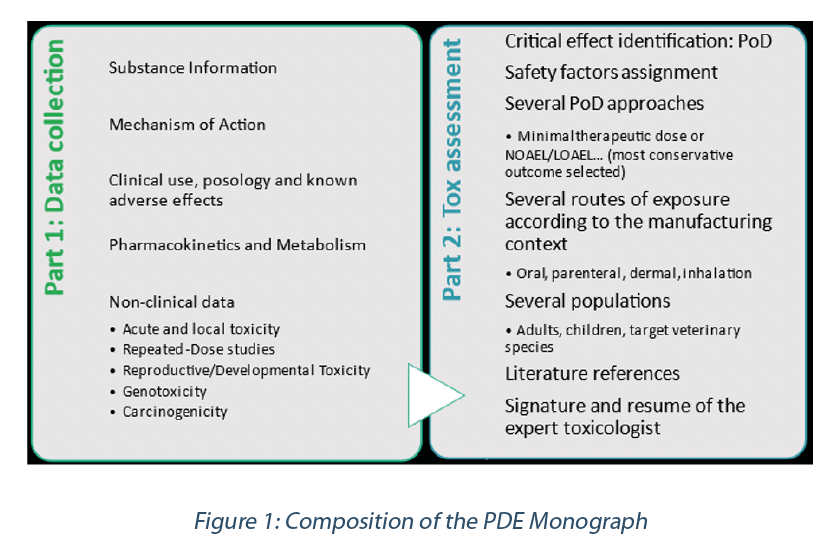
Toxicological approach to define the PDE for your cleaning validation process. - A3P - Pharmaceutical & Biotechnology Industry

Permitted Daily Exposure (PDE) Certification Company | Acceptable Daily Exposure (ADE) PDI Value Derivation & Calculation